FileOpen for Healthcare & Life Sciences

Privacy and Security for Healthcare Information
The numerous regulatory and privacy frameworks that govern HLS organizations mandate controlled document distribution. Documents must be controlled and tracked via auditable systems both inside and outside the institutional environment. Medical and scientific computing environments bring exceptional security requirements, with "locked-down" desktop environments that do not permit software installation by end-users. These challenges are unique and require flexible solutions that can work within the larger systems used by HLS organizations.
Public/Private Partnership
FileOpen's solutions are operating today in hospitals, government health institutes, pharmacutical research centers, medical information publishers, and other medtech environments.
- Protect patient health records according to HIPAA
- Secure clinical trial documentation
- Protect patent applications and other IP
Prevent saving a local copy
Enable offline viewing for limited periods
Expire access after a fixed date

Use Cases
Implement controlled access to document collections | Manage secure review of research proposals | Segment and protect regulatory filings | Ensure confidentiality in patient communications
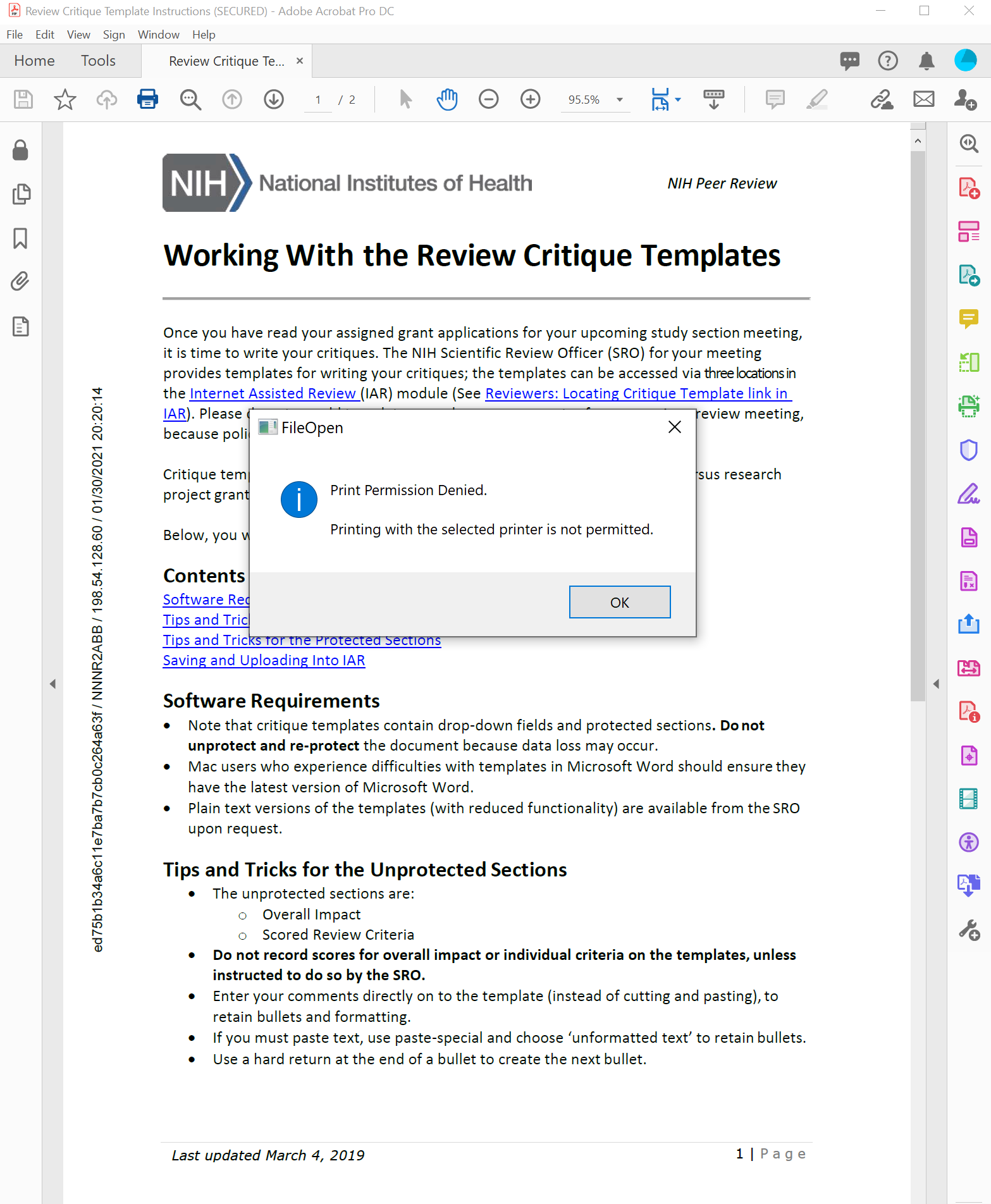
Example Document
FileOpen DRM is frequently implemented to control the printing of PDFs and to prevent users from using the print function to create unprotected digital copies, as shown here.
